KATHAROMETER TECHNOLOGY

A thermal conductivity gas sensor, also known as a katharometer, is a common technology allowing measurement of the concentration of flammable gases. Its main advantage over traditional catalytic sensors (or pellistors) is their ability to measure levels of concentration above the Lower Flammability Level (LFL). However, traditional thermal conductivity gas sensors suffer from high power requirements and demand high level of precision and craftsmanship in manufacturing, and this has so far prevented a widespread use in industrial safety applications.
Thermal conductivity gas detection
Thermal conductivity sensors measure the concentration of gases having thermal conductivity significantly different to a reference gas (normally, air), between 0 and 100% volume. Gases with thermal conductivity similar to air, notably oxygen, nitrogen and CO, in fact, cannot be measured using this technique.
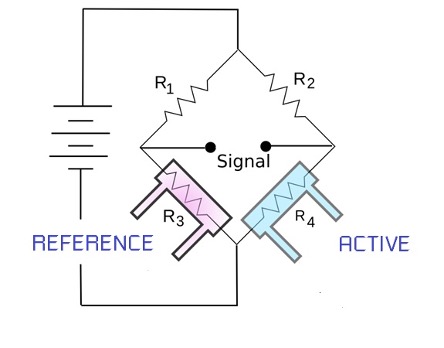
A thermal conductivity gas sensor is formed by two dies – one freely exposed to the target gas (the active die) and the other sealed in a chamber containing air (the reference die). Both dies are heated using constant current and run in a classic Wheatstone bridge circuit. Thermal conductivity sensors measure the change in heat loss of the active die in the presence of the target gas. In fact, when the active die is exposed to a gas with thermal conductivity different to that of air, the rate of heat loss from the die will change and so will its resistance. This change is compared with the resistance of the reference die.
For the reasons detailed above, thermal conductivity sensors are subject to specific cross sensitivity with other gases whose thermal conductivity is also significantly different from that of air. Therefore, thermal conductivity sensors perform best in applications where interfering gases are absent, or their cross sensitivity is within the acceptable margin of error required by the application.
Advantages
Thermal conductivity sensors are most effective when detecting gases with low molecular weight, which correspond to greater thermal conductivity – such as Hydrogen, possessing possesses the highest thermal conductivity of all known gases, and Helium.
Thermal conductivity sensors, unlike catalytic bead sensors, covers the broadest range of detection, working well from ppm level, up until 100 % volume. This is because they can operate without the presence of Oxygen.
Katharometer Gas Technology provides far better long-term stability than sensors that are triggered by chemical reactions that eventually cause the sensor to degrade. Thermal conductivity gas sensors, in fact, do not involve physical or chemical changes in the sensor. This, coupled with outstanding resistance to poisoning, results in far greater operating lives than for traditional technologies.
NET MEMS membrane-based sensor offers a far greater resistance to mechanical shocks when compared to traditional catalytic or thermal conductivity sensors.
For safe operation and to minimize power consumption, the sensor is excited with a pulsed waveform (400 ms on and 1,000 ms off), resulting in a heater temperature that is almost the same as the ambient.
Another key factor is the fast response time of the sensor (< 1.4 s), the only limiting factor being the time required for changes in the measurement resistor.